|
10/20/2014 0 Comments What’s in my laptop?By Mary Gallagher Welcome to What’s in my…?, where Mary takes a look at the mineral resources that go into the stuff we use every day!
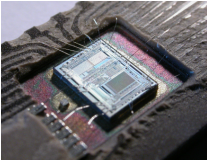
Silicon is great for microchips because it’s a semiconductor, which means that it can be “doped” (the real name for a process where impurities are introduced into the pure silicon) to make it more conductive, making it easy to shape the electrical current within the electronic device.[1] Those immensely complex microchips are made from something that you may have had stuck in your hair all summer: SiO2, quartz, regular old sand. The sand is mined in open pit mines like this one in the Czech Republic:  Although silicon makes up 27.7% of the earth’s crust and sand may seem like a renewable resource, sand mining has several concerning environmental impacts. Aqueous sand mining (like in Maharashtra and Goa, India) disrupts the natural course of rivers, allowing the intrusion of salt water during tidal rises and leading to erosion and habitat destruction.[2] In the past few years there has been a movement to put more environmental regulations on sand mining in India to protect fisheries and properties from erosion, especially because much of the mining is done illegally on public or protected lands.[3] Many of those miners are former fishermen in an area where fisheries have already been decimated, and who rely on the high demand for sand mining to make a living. After being mined, sand is transformed into microchips in fabrication plants known as “fabs.” In the fab, sand is melted down into 99.9999% pure silicon and etched into to create a complex integrated circuit.[4] After that, aluminum or another conducting metal is added, and the chip is tested and shipped off to be embedded in our laptops or pacemakers or ATM machines. As microchips are embedded in everything from running shoes to fishing reels, the demand for silicon will only rise over the next few decades. Hopefully, the same spirit of technological advancement that is promoting the use of microchips will encourage the advancement of more sustainable environmental practices in areas where silicon is sourced and mined. [1]http://www.computerworld.com/article/2576786/computer-hardware/making-microchips.html [2]http://www.cbd.int/kb/record/sideEvent/2682?Record&Event=COP-11) One species of crocodile, the gharial, has been nearly driven to extinction by sand mining. (http://www.ircf.org/programs/gharial/gharial-threats/) [3]http://www.business-standard.com/article/pti-stories/police-team-attacked-by-illegal-sand-miners-opens-fire-114092401197_1.html [4]http://www.computerworld.com/article/2576786/computer-hardware/making-microchips.html)
0 Comments
Leave a Reply. |
WELCOME, UMICH SCIENTISTAS!
CAMPUS PICS
WHAT'S NEWUPCOMING EVENTSPAST POSTS
October 2022
SORT BY TAG |
The Scientista Foundation, Inc. All Rights Reserved © 2011-2021 | Based in NY | [email protected]
The Network for Pre-Professional Women in Science and Engineering
The Scientista Foundation is a registered 501(c)(3) -- Donate!
The Network for Pre-Professional Women in Science and Engineering
The Scientista Foundation is a registered 501(c)(3) -- Donate!




 RSS Feed
RSS Feed