|
11/24/2015
Fixing the Microbial Tally in Our Guts
By Uma Chandrasekaran
Greek philosopher Thales once said “A sound mind in a sound body”, suggesting that mental and physical well-being are closely linked. The recent discoveries made by scientists on the gut microbial community in humans have made us believe, “A sound gut for a sound body”. ‘Gut microbes influence obesity’, ‘Gut bacteria and cancer incidence’, ‘Gut bacteria guide your mind’, ‘Diabetes start in your Gut’, ‘Gut bacteria signal Asthma Risk’ and the list goes on. In all, gut microbial community or intestinal microflora is emerging as a major pivot between health and disease.
It turns out our intestines are home to approximately a hundred trillion bacteria. In other words, bacteria outnumber human cells in our body by 10:1. But, why? What do these bacteria do? Based on research studies, scientists suggest that humans have a mutualistic relationship (live and let live!) with the gut bacteria. The microorganisms enjoy BnB (bed and breakfast) inside our guts and in turn help us in digesting complex carbohydrates, production of fatty acids and synthesis of vitamins. Not just that, the resident commensal bacteria protect us from pathogenic (disease-causing) bacteria through competitive exclusion (Trespassers Beware!).
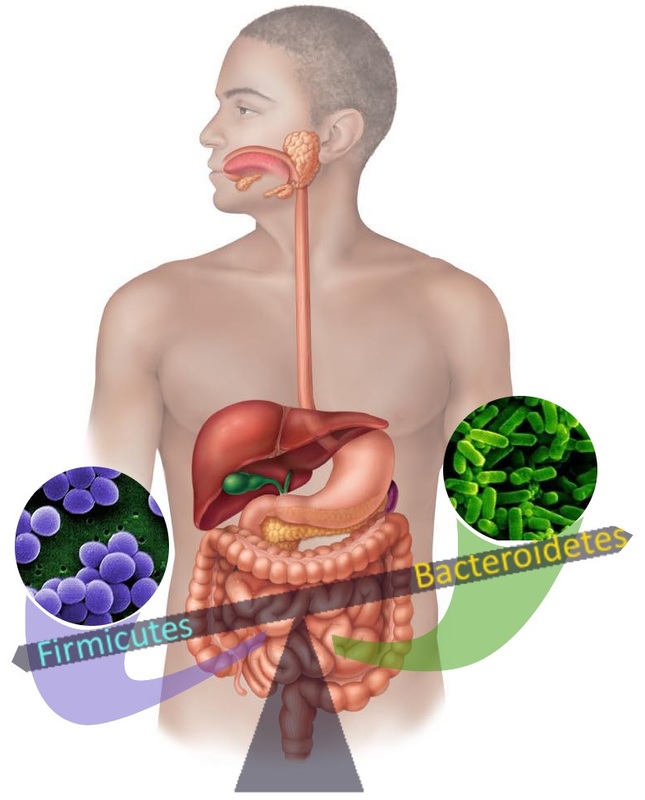
The trillions of bacteria in our gut mainly belong to two superkingdoms: Bacteriodetes (48%) and Firmicutes (51%). Bacteriodetes are predominantly Gram-negative, non-spore forming and rod shaped while Firmicutes are Gram-positive, spore forming and can be found in both round or rod-like forms. As mentioned previously, members of both the Firmicutes and Bacteriodetes family benefit the host through fulfilling vital functions like digestion of dietary carbohydrates. A fine balance among the bacterial communities in our guts is required to maintain health and well-being. Turns out that changes in the number, type and/or the location of the gut microflora, causing alterations in the fine balance can have serious health consequences. For example: Higher proportion of Firmicutes to Bacteriodetes have been shown by researchers to correlate with obesity, and inflammatory responses. Also, low numbers of Firmicutes like Lactobacillus species promote expansion and colonization of disease causing bacteria like Clostridium difficile, which causes infectious diarrhea. In addition, the spread of Bacteriodetes like Bacteroides fragilis from gut to the blood stream has been reported to cause inflammation. What causes the alterations in the gut bacterial composition (dysbiosis)? Diet and use of antibiotics seem to be the two main culprits influencing the gut flora. For example, high fat diet (read Supersize Me diet) promotes an increase in Firmicutes with a relative reduction in Bacteriodetes.
Antibiotics are the mischief mongers in the gut, even though they were the wonder drug that brought us back from the clutches of death. So, even though they can help cure infections by killing bacteria, they are also indiscriminate killers that can kill off innocent bystanders like the commensal gut bacteria. With the loss of the sentinel-bacteria in the gut, the field is wide open for opportunistic pathogens like Clostridium difficile to colonize and wreak havoc. The widespread use of antimicrobials in personal care products and overuse of antibiotics has contributed immensely to dysbiosis. So much so that, more than 450,000 Americans suffer from antibiotic associated diarrhea (AAD) and the ensuing C.difficile infection every year.
So what now? Should we stop using antibiotics? Definitely judicious use of antibiotics will help with the need of the hour. In addition, scientists are trying to establish homeostasis (re-populate the gut bacteria and maintain their relative ratio) that antibiotics have destroyed through hijacking the bacterial communication system. Bonnie Bassler of Princeton university, discovered that bacteria communicate with each other through a process called quorum sensing. In this process, bacteria produce chemical messengers called autoinducers. As the number of bacteria increases, so does the amount of autoinducers they produce. Once the level of autoinducers reaches a critical threshold, they trigger downstream signaling cascades resulting in collective behaviors, such as biofilm formation, motility, virulence factor expression etc. Hence, quorum sensing is a communication tool used by bacteria acting in synchrony that bestows them with a distinct advantage. For example, when there are only few pathogenic bacteria in the host, these bacteria together release very little autoinducer. Once the population of pathogenic bacteria reaches a substantial number, the collective autoinducer produced by pathogenic bacteria triggers the expression of genes that helps them colonize the host and feed their pathogenic lifestyle. Evidently, novel molecules aimed at modulating/interfering with the quorum sensing behavior canserve as therapeutic targets. One such autoinducer observed throughout bacterial kingdom is called autoinducer-2 (AI-2). As published recently in Cell Reports, Karina Xavier and her team from the Instituto Gulbenkian de Ciencia (IGC) in Oeiras, Portugal, reported that manipulating the levels of AI-2 in the gut following antibiotic treatment might help restore the microbial balance. To test their hypothesis the researchers created the scene first - they treated mice with the antibiotic streptomycin for 28 days. As expected, antibiotic treatment resulted in significant decrease in the total gut bacteria of the mice and a major shift in the balance between Firmicutes (from 43% to 0.7%) and Bacteriodetes (from 48% to 90%). Next, the researchers introduced genetically modified E.coli strains into the mice gut two days after the start of the antibiotic treatment. Mutant E.coli strains were engineered to be streptomycin resistant and were capable of depleting environmental AI-2 (control group) or producing high amounts of extracellular AI-2 (experimental group). Different E.coli strains were able to colonize the mice gut to the same extent owing to the space freed up by streptomycin treatment. The result: on day 28, following streptomycin treatment, mice that had high levels of AI-2 in their gut had 3-6 fold more Firmicutes compared to those with low levels of AI-2. In other words, using AI-2 the researchers were, to a certain extent, able to negate the detrimental effect of antibiotic treatment on Firmicutes. “We attempted to establish homeostasis in the gut after dysbiosis and these results suggest we are on the right track,” said Jessica Thompson and Rita Olivera, the study’s first authors. They further added, “We would like to know the mechanism by which AI-2 is regulating the effect and how we can apply this to humans.” This is one of the first studies highlighting the potential of manipulating bacterial communications within the gut to restore homeostasis. Thrilled by the success of their efforts and brimming with hope, Jessica and Rita said, “It would be interesting to study the effect of AI-2 or other quorum-sensing molecules on the gut microbiome in the absence of antibiotic treatment.” At present, the research team is performing additional studies involving feeding mice with a drug containing AI-2. Dr. Karina Xavier told The Scientist “Now that we know increasing AI-2 has positive effect, it makes sense to try to add it directly”. Looks like hijacking bacterial communication might turn out to be an antidote to antibiotics. While scientists are trying to restore the microbial balance in our guts, we can definitely do our part to maintain a balanced microbial environment in the gut by eating a:
Are you hungry? Grab a carrot and some yogurt. Now you know, it is better you choose your bugs than they choose you! -- Image Sources: Blue bacteria Green bacteria Human intestine About the Author
Uma Chandrasekaran, recently completed her post-doctoral fellowship in autoimmunity and made the transition from academia to the business side of science. She always loved communicating science and recently discovered the Scientista platform. When she's not wrapping her brain around a scientific concept, she's either running behind a "mini-me" or cooking.
Comments? Leave them below! |
Archives
February 2023
DiscovHER BlogScientista DiscovHER is a blog dedicated to discovHERies made by women in science. Follow us for links to the latest resHERch! Categories
All Alexandra Brumberg Amy Chan Avneet Soin Chemistry Diana Crow Engineering Health/medicine Indulekha Karunakaran Iqra Naveed Johanna Weker Lidiya Angelova Michael Clausen Mind Brain And Behavior Muhammad Hamza Waseem Nikarika Vattikonda Opinion Prishita Maheshwari-Aplin Technology Uma Chandrasekaran |
The Network for Pre-Professional Women in Science and Engineering
The Scientista Foundation is a registered 501(c)(3) -- Donate!

 RSS Feed
RSS Feed